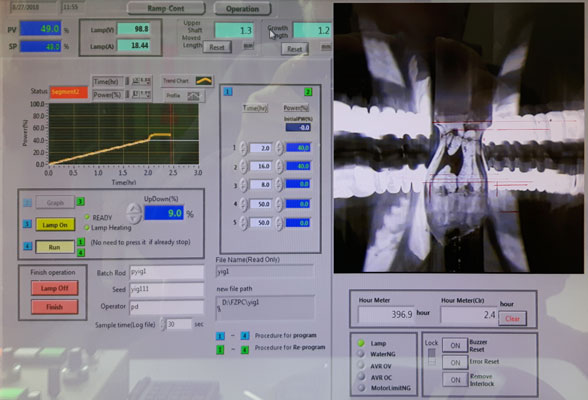
20 years of crystal growth, production and manufacturing!




SurfaceNet GmbH located in Rheine (Germany) was founded in 2003. SurfaceNet offers a wide range of products for the scientific and industrial world of electronics and optics. Our main target is the so called crystal world.
We produce materials like raw crystals, substrates with surfaces in highest finish and components made out of single crystals like substrates, deposition materials like targets and a wide range of machinery materials.
Most of the processed crystals are synthetic crystals made by man but we work also with natural materials as not every chemical composition is available as synthetic crystal.